The UK’s National Institute of Health and Care Excellence (NICE) has published an update to its methods and processes for health technology appraisal. The update comes after a year long review and includes a number of significant changes to how innovative medicines will be evaluated for pricing and reimbursement in the UK.
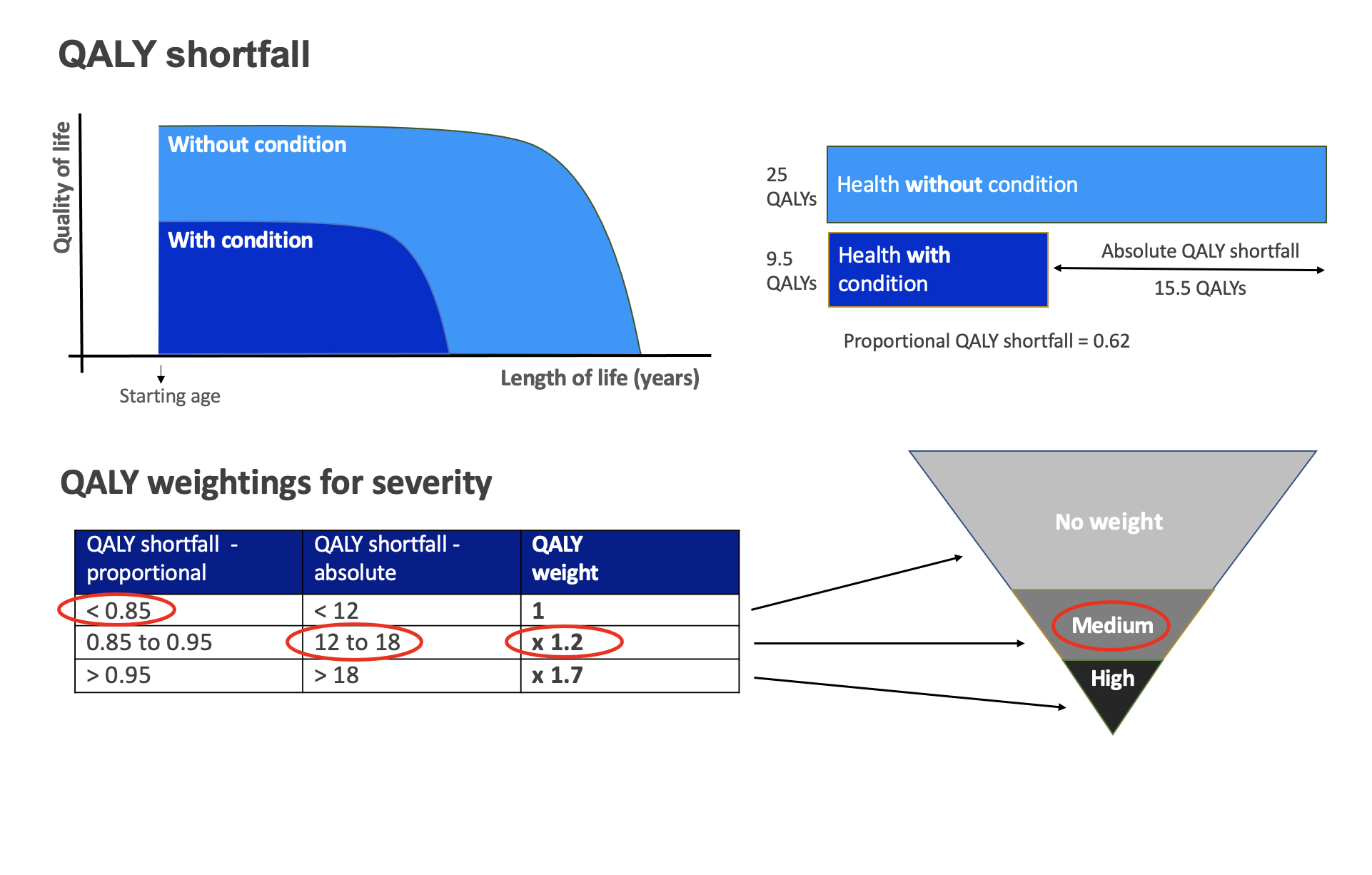
One key change to the NICE methods is the introduction of a “severity modifier”. Previously all QALYs were considered of equal value in NICE decision-making, with the exception of “end-of-life” therapies and treatments for ultra-rare diseases assessed via the highly specialised technologies (HST) track. NICE is now introducing a severity modifier, which will place increased value on QALY gains in severe diseases.
For conditions considered more severe, QALY gains will be upweighted by a multiplier of 1.2 or 1.7. Eligibility for the multiplier will be determined by lifetime QALY shortfall, absolute or proportional, for the eligible patient population compared to the general population.
This change will make demonstration of burden of disease and evidence on disease natural history a much higher priority for conditions that are potentially eligible for the severity modifier.