- Details
A tale of two winters: what a difference a vaccine makes
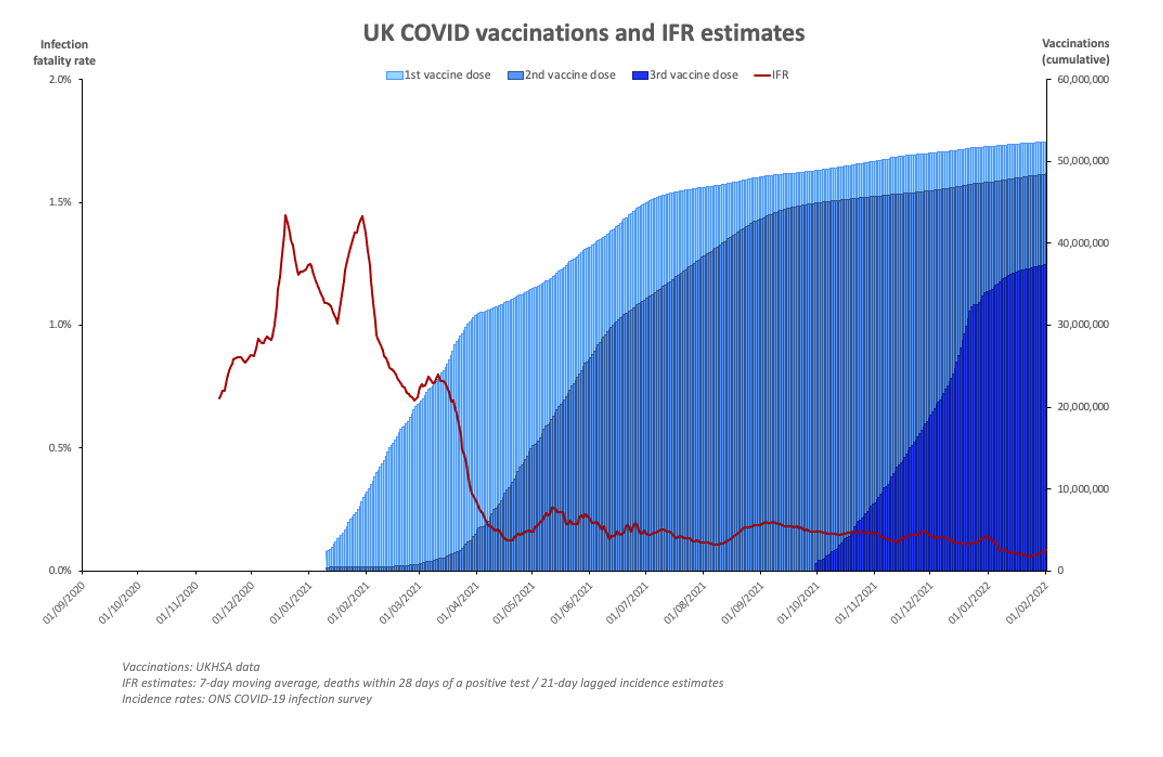
This post was originally published without text. During the pandemic often alternative interpretations were conjured from the same data, depending on the point of view of the "analyst". Some times the data are truly compelling and its best to let them speak for themselves.
- Details
NICE severity modifier
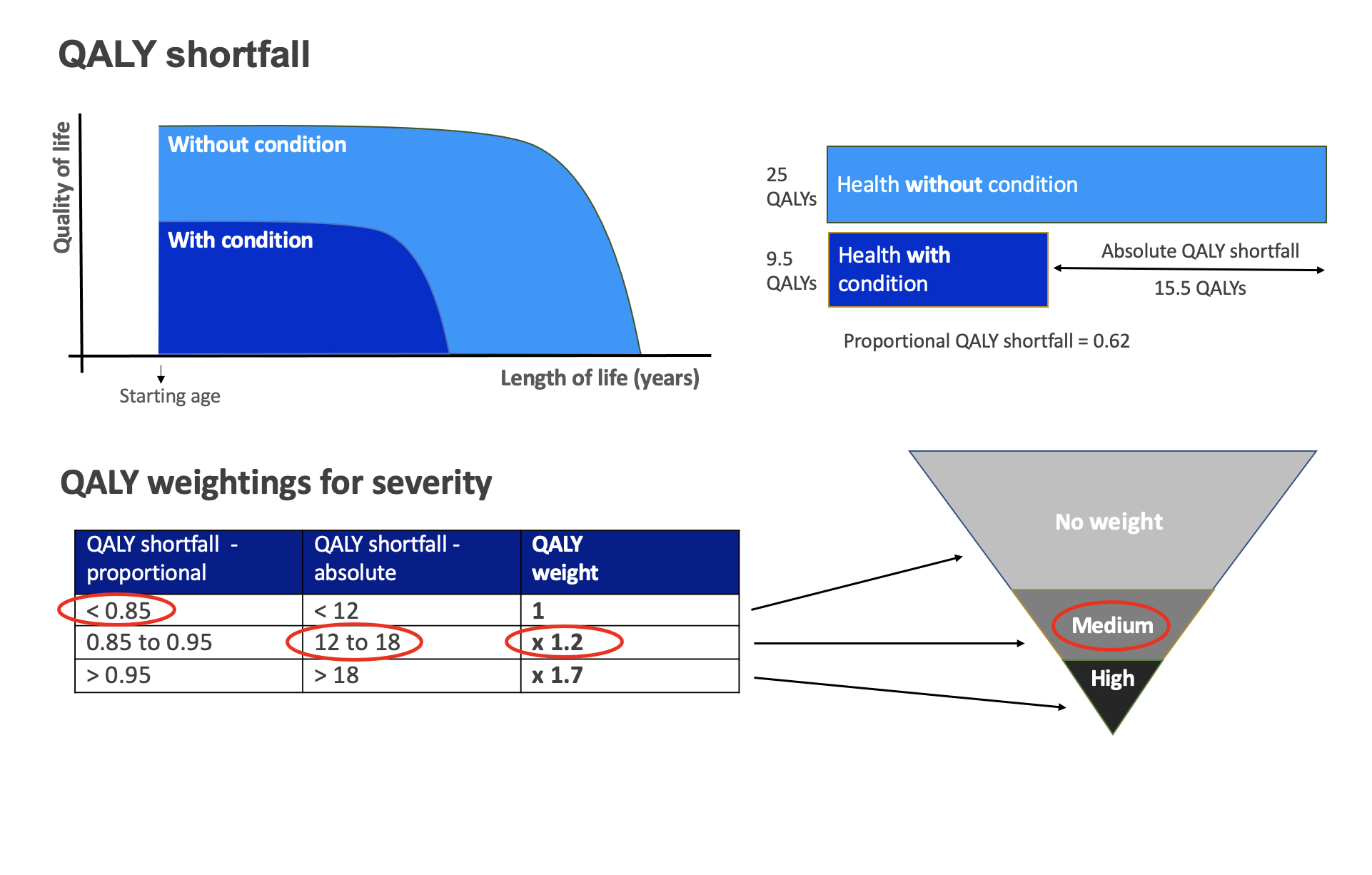
The UK’s National Institute of Health and Care Excellence (NICE) has published an update to its methods and processes for health technology appraisal. The update comes after a year long review and includes a number of significant changes to how innovative medicines will be evaluated for pricing and reimbursement in the UK.
- Details
ESMO editorial: alternative models for manufacture of CAR-T therapy

The EU’s “hospital exemption” rule permits the manufacture and use of CAR-T therapy in academic centers, with member state regulatory oversight. This has been proposed as an alternative to the commercial model for CAR-T development, with global clinical trials and centralized regulatory approval, manufacturing and pharmacovigilance.
- Details
Multiple myeloma therapy: untangling treatment sequences
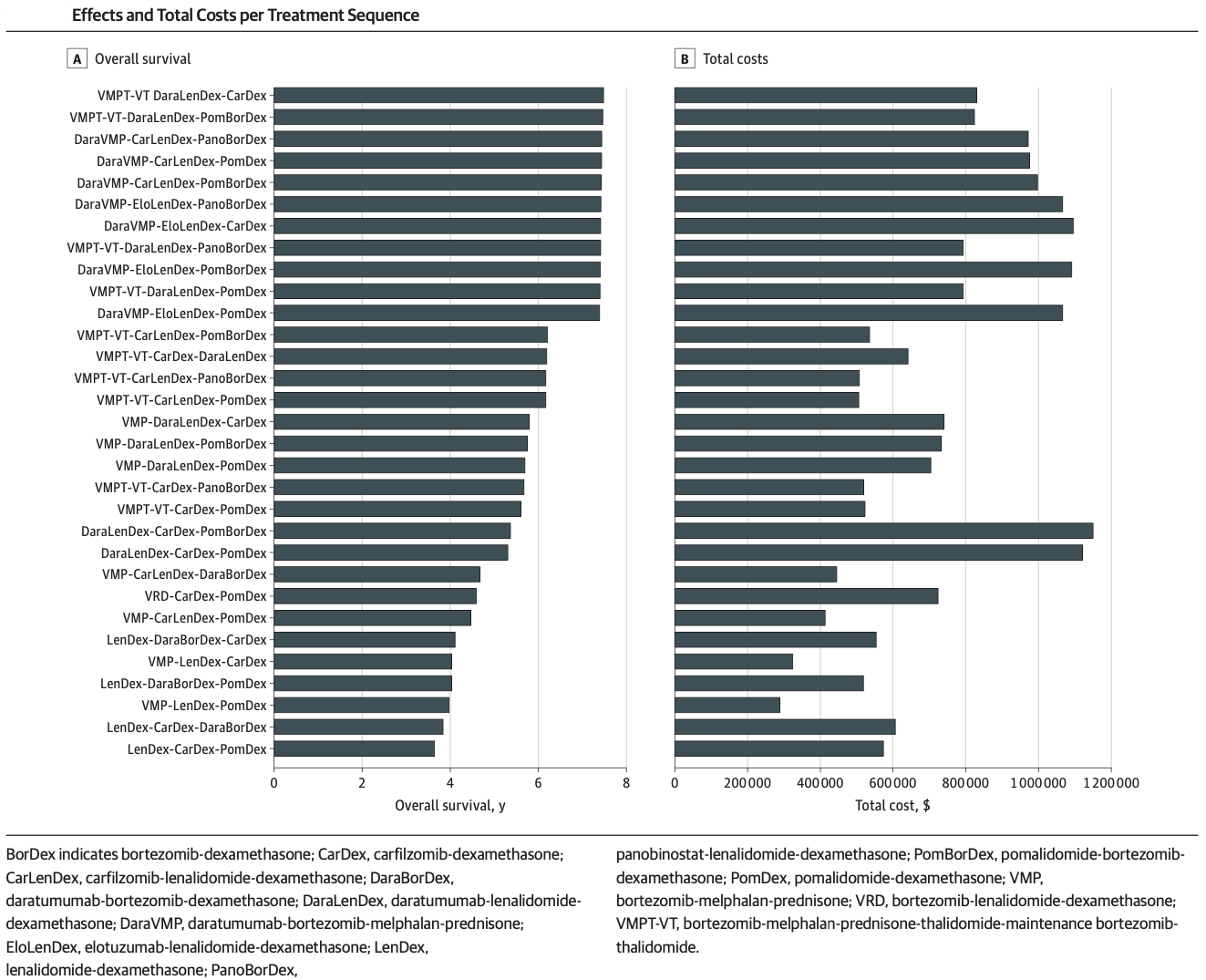
The last 5 years has seen a dramatic evolution in treatments for multiple myeloma. Previously, bortezemib, lenalidomide and pomalidamide, in combination with steroids and used sequentially, was the mainstay of treatment for transplant ineligible patients. With the introduction of carfilzomib, panobinostat, elotuzumab, daratumumab and ixazomib as add-ons to existing therapies, there is now a much large number of possible combinations and sequences.







