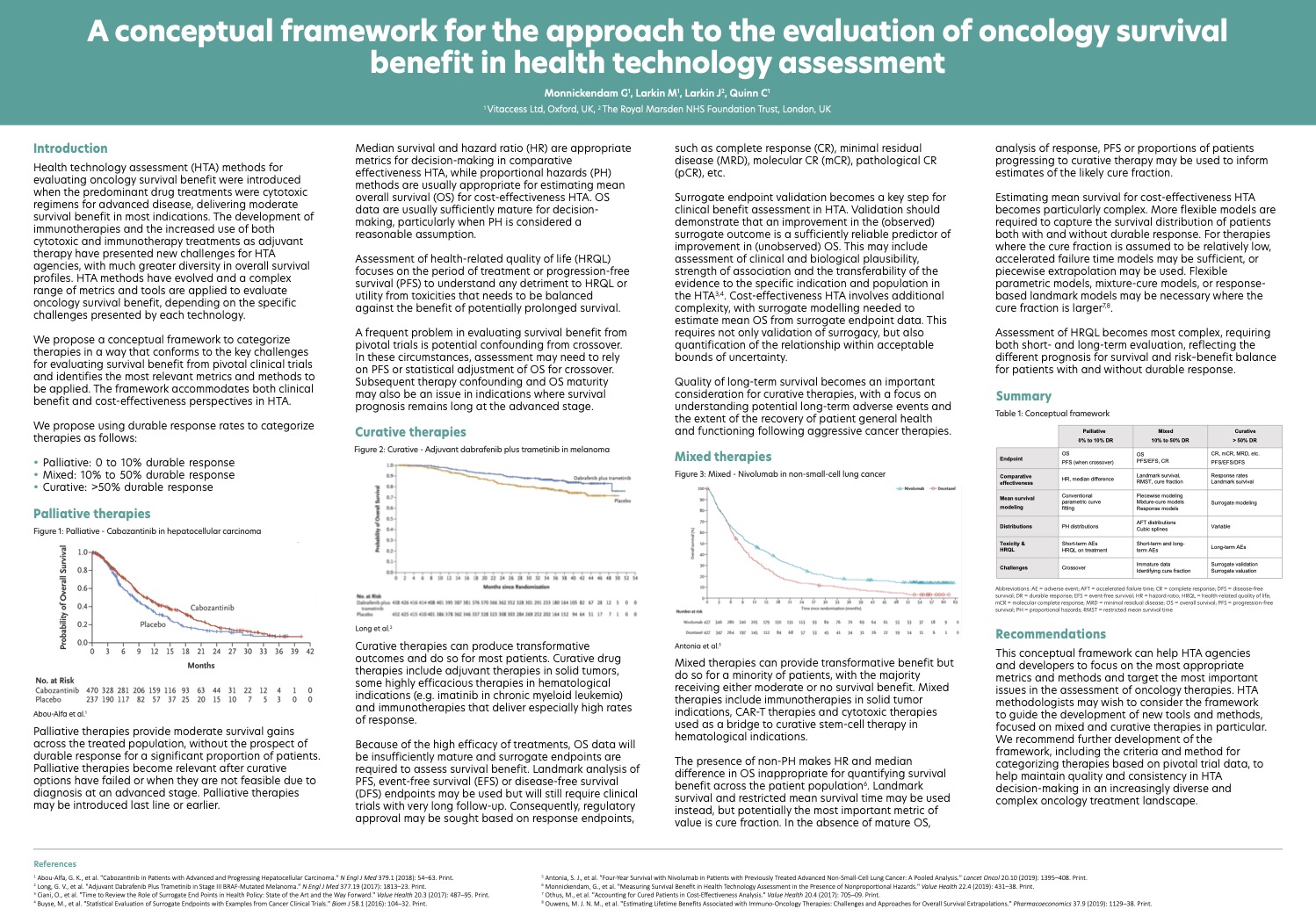
HTA methods for evaluating oncology survival benefit were introduced when most drug treatments were cytotoxic regimens for advanced disease, delivering moderate survival benefit. Immunotherapies now offer the potential for transformative benefit in many indications. Adjuvant drug therapies with curative intent in earlier disease stages are also expanding. The increased diversity in survival benefit delivered by new technologies adds complexity and presents new challenges for HTA evaluation of oncology therapies.
Different metrics, methods and issues are now relevant for HTA and the appropriate toolkit needs to be selected for assessments. Validation of surrogate endpoints and quality of long-term survival become particularly important for demonstrating the value of potentially curative therapies.
Surrogate endpoints are a current “hot topic”. Oncology survivorship - understanding the experience and quality of life of people who achieve long-term survival with oncology treatments – is an under-researched area, but one we expect to become increasingly important.