- Details
Evaluating survival benefit for HTA
HTA methods for evaluating oncology survival benefit were introduced when most drug treatments were cytotoxic regimens for advanced disease, delivering moderate survival benefit. Immunotherapies now offer the potential for transformative benefit in many indications. Adjuvant drug therapies with curative intent in earlier disease stages are also expanding. The increased diversity in survival benefit delivered by new technologies adds complexity and presents new challenges for HTA evaluation of oncology therapies.
- Details
Innovative pricing in France: a sceptical CEPS
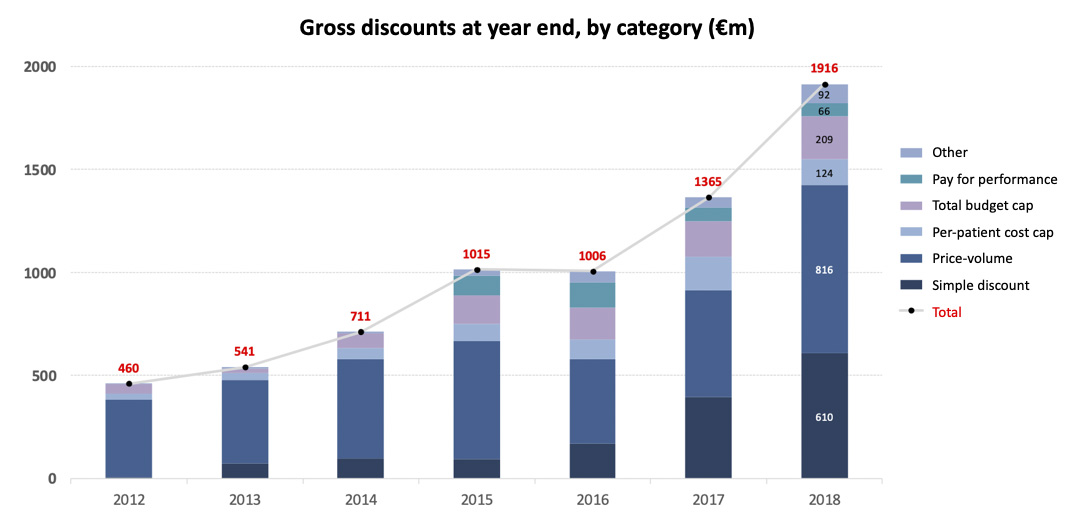
The 2018 annual report of the Comité Economique de Produits de Santé (CEPS), provides valuable insights into France’s stance on innovative pricing schemes for drugs. CEPS has published the annual value of rebates, split by type of pricing scheme.







